Sep 10, 2025 | Blog Post
Diffuse pleural mesothelioma remains a uniquely challenging malignancy. Its growth pattern undermines conventional imaging, its biology yields scant circulating tumor signal, and debate exists around the value and timing of surgery. Against this backdrop, we asked two simple questions of central practical importance and clinical significance. First, can immune checkpoint blockade be given before and after surgery without derailing the operative plan or compromising safety in patients with resectable pleural mesothelioma? Second, can an ultra-sensitive, tumor-informed liquid biopsy provide a clinically meaningful molecular read-out of peri-operative immunotherapy outcomes, early enough to guide therapeutic decision making?
Patients with good performance status and multidisciplinary confirmation of operability received either neoadjuvant nivolumab alone or combined with a single dose of ipilimumab. All patients who underwent resection also received post-operative therapy, including adjuvant nivolumab for one year per protocol. The co-primary endpoints were feasibility, defined a priori as proceeding to surgery without protocol-defined delay, and safety, captured by dose-limiting toxicities during the neoadjuvant window. Progression-free and overall survival were evaluated as exploratory outcomes alongside tumor-informed, whole-genome sequencing liquid biopsy, measuring circulating tumor DNA (ctDNA) at baseline, through neoadjuvant therapy, immediately pre-operatively, and again post-operatively.
The clinical verdict on feasibility and safety was clear. More than four-in-five patients in each arm reached the operating room as planned, and complete macroscopic resection was achieved in most cases. Immune-related toxicities aligned with expectations for immunotherapy agents, and adjuvant nivolumab was generally well-tolerated. These findings address the core, practical anxiety that neoadjuvant immunotherapy might derail operability, which it did not.
Efficacy signals, while exploratory in this phase 2 clinical trial setting, were encouraging and coherent. Median progression-free and overall survival favored the combination of nivolumab with ipilimumab over nivolumab alone, and a meaningful fraction of patients in the combination arm remained alive and recurrence-free at the time of data analysis. One should avoid over-interpreting arm-to-arm contrasts in this small, sequentially-accrued study, but the pattern – durable control in a subset without dramatic radiographic responses – foreshadows the central translational lesson of this work.
That lesson is that, in mesothelioma, cell-free tumor DNA detection in the bloodstream is a more reliable indicator of treatment effect than tumor measurements on imaging. Conventional response rates by modified RECIST were modest, as expected in this pleural surface-spreading cancer type, which often thins rather than shrinks; yet the ctDNA assay drew a much sharper picture. By leveraging genome-wide mutation profiles from each patient’s tumor, and utilizing a machine learning framework to suppresses technical noise in cell-free DNA, we were able to detect extraordinarily low tumor levels and track ctDNA dynamics over time. Two clinically intelligible patterns emerged.
First, detectable ctDNA during the neoadjuvant window correlated with the feasibility endpoint at the heart of this trial. Patients who ultimately could not complete surgery – either because their disease progressed between planning and incision, or because the tumor proved unresectable intraoperatively – showed persistent or rising ctDNA levels, while still being nominally eligible on imaging. This is the kind of actionable information that can promptly redirect specific patients to alternative treatment strategies.
Second, ctDNA served as an indicator of long-term progression-free survival. Patients with undetectable ctDNA by the end of neoadjuvant therapy and immediately before surgery experienced substantially longer progression-free survival than those with residual ctDNA; this same pattern held, often more strongly, when one looked at two-timepoint dynamics. Significant drops in tumor fraction from baseline, or persistently undetectable ctDNA throughout the neoadjuvant course, were observed in patients most likely to attain durable control after surgery; additionally, post-operative re-emergence of ctDNA predicted disease recurrence. Importantly, these molecular trajectories frequently diverged from scan-based impressions. In other words, ctDNA captured the direction and velocity of disease progression and therapy response when imaging techniques could not.
It is worth pausing on what, precisely, we can and cannot claim based on these data. The notion that ctDNA is prognostic, i.e., that it stratifies patients by risk independent of treatment choice – is strongly supported here, since those without detectable ctDNA at key junctures attained better outcomes. Whether ctDNA is also predictive, i.e., whether its clearance specifically marks benefit from neoadjuvant checkpoint blockade, is a more nuanced claim in a non-randomized phase 2 study without a non-immunotherapy control arm. The temporal coupling of ctDNA reduction to neoadjuvant dosing, and the association with improved clinical outcomes, together make a persuasive case that the ctDNA signal is treatment-linked. However, definitive proof will require larger, prospective trials, adequately powered to prove the clinical benefit of a ctDNA-guided adaptive therapy.
Two practical implications follow immediately. First, the trial establishes that neoadjuvant checkpoint blockade can be considered among curative-intent therapy options for resectable mesothelioma without jeopardizing surgery. Second, ctDNA residual disease after neoadjuvant immunotherapy and prior to surgery – offers a crisp, quantitative, molecular read-out that may refine individual care. Clear molecular response supports proceeding to resection, while a persistent ctDNA signal should trigger a re-consideration of medical management. After resection, reappearance of ctDNA should prompt intensified surveillance and the swift deployment of systemic therapy.
No early-phase study is without limitations. Arms were accrued sequentially, and the participating sites brought deep surgical expertise that not all centers can immediately replicate. Mesothelioma’s low mutational burden and locoregional spread will always challenge liquid biopsy analytical and clinical sensitivity. Yet even within these constraints, the core conclusions hold; perioperative immunotherapy is feasible and safe, and ctDNA provides an accurate and timely account of tumor burden dynamics and therapeutic response – something imaging alone has not reliably delivered in this setting.
Taken together, the purpose of this trial was to determine whether perioperative immune checkpoint blockade is practical in resectable mesothelioma, and whether an ultra-sensitive tumor-informed ctDNA assay can serve as an early, clinically-useful biomarker. The result is yes on both counts. The therapy was feasible and safe, and the liquid biopsies were not merely measurable, but clinically informative, identifying patients likely to attain clinical benefit, signaling those at risk of surgical attrition or early recurrence, and offering a path to more adaptive, individualized, and precise perioperative care. As the field moves to larger studies, these findings provide a clear blueprint; keep immunotherapy in the perioperative conversation, and let ctDNA, measured carefully and interpreted judiciously, guide the rhythm of that dialogue.
Dive deeper and read the full study here: https://www.nature.com/articles/s41591-025-03958-3
Aug 18, 2025 | Blog Post
Precision oncology has fundamentally reshaped the treatment paradigm within the field, with individualized biomarker-matched therapies enhancing the arsenal oncologists use to manage cancer.
For the evolving ecosystem of precision oncology to advance toward a nationwide integration, efforts should focus on expanding access to genomic expertise, molecular tumor boards, and clinical trial infrastructure – resources that remain scarce outside academic centers. There thus arises a need to shift the strategic approach to advancing precision oncology so that it emphasizes not only technological and pharmacological progress but also clinical utility and broader accessibility.
Significant efforts have been made toward establishing comprehensive molecular profiling in first-line decision-making, where clinically appropriate. Non–small cell lung cancer (NSCLC) has long exemplified the integration of baseline molecular testing; where genomic alterations such as EGFR mutations, ALK and ROS1 rearrangements, MET exon 14 skipping, BRAF V600E mutations, and PD-L1 expression levels are systematically assessed to guide personalized therapy. Similarly, in breast cancer, estrogen receptor (ER) and progesterone receptor (PR) expression, and epidermal growth factor receptor 2 (HER2) expression/amplification are predictive biomarkers evaluated at baseline. Furthermore, tumor genotyping for PIK3CA and ESR1 mutations has become clinically actionable, with FDA approvals of specific drug classes for these biomarker-defined subsets of hormone receptor-positive breast cancer. These models illustrate that when actionable biomarkers are implemented, testing informs therapeutic decision-making and improves clinical outcomes.
In terms of real-time monitoring of tumor burden and therapeutic response, liquid biopsy analyses have emerged as paradigm-shifting technologies; longitudinally assessing circulating tumor DNA (ctDNA) dynamics and evaluating residual disease across stages and therapies. Liquid biopsies also allow for tracking tumor evolution, detecting emerging resistance, elucidating mixed responses or stable disease, and identifying recurrence before it becomes radiographically or clinically apparent.
Establishing precision oncology as the standard of care, however, requires two key elements: technology to advance cancer discovery and education to scale its impact. By providing expert molecular interpretation and implementing decision-support tools in community clinics, we can expand access and enrolment in biomarker-matched therapies and clinical trials.
At this precise intersection – between education and technology – we are building a precision-oncology decision-support platform embedded within the Johns Hopkins Molecular Tumor Board to standardize evidence assessment and expand equitable access to expert interpretation and recommendations. In line with the scope of the NCI Community Oncology Research Program – which bridges clinical trials and care delivery research with community clinics – we envision that our initiatives will help operationalize precision oncology and create clear on-ramps to clinical trial screening and enrolment.
Outreach programs tailored for community oncologists, improved referral networks, and regionally or virtually hosted case-based molecular tumor boards (MTBs) have further democratized access to genomic expertise and improved clinician confidence in navigating molecular findings. Importantly, these educational models should be sensitive to workflow realities in high-burden settings and emphasize the clinical utility of precision oncology approaches. Expanding genomic literacy beyond the physician level to include nurse navigators, genetic counsellors, and case managers can further support patient-centered education, and increased engagement in shared decision-making processes, including clinical trial participation.
Internationally, promising models are emerging. Regional MTBs supported by international consortia have demonstrated feasibility and clinical benefit even in resource-constrained settings, providing a blueprint for scalable transnational and supranational precision oncology frameworks.
The future of oncology depends not only on identifying new biomarkers and developing advanced targeted therapies, but also on breaking down the educational and logistical barriers that restrict their access. Through the meaningful work of precision oncology groups, including our own at the Johns Hopkins MTB, we are hopeful that soon precision oncology will evolve from a specialized innovation into an accessible standard, embedded across all levels and stages of the cancer care continuum.
Jul 27, 2025 | Blog Post
Over the past decade, significant progress has been achieved in treating lung cancer, particularly for individuals with non-small cell lung cancer (NSCLC). In particular, the development of immune checkpoint inhibitors – which harness a patient’s immune system to fight cancer – has resulted in significant improvements in patient outcomes. Immune checkpoint inhibitors work by blocking inhibitory molecules that cancer cells use to evade immune surveillance, enabling the patient’s immune system to target cancer cells. This therapeutic approach is entirely unlike chemotherapy, which targets all rapidly dividing cells and thus has the potential to target some healthy cells – e.g., hair cells and cells in the lining of the digestive tract.
Still, not all patients attain clinical benefit from these novel immunotherapies. Some lung cancers harbor an “immunologically cold” phenotype, meaning they are more likely to evade immune recognition and elimination by the immune system even when treated with immune checkpoint inhibitors. For example, tumors with fewer somatic mutations, which are considered to have a low tumor mutation burden (TMB), and tumors with no PD-L1 expression, generally show a poorer response to immunotherapy. This raises the question of how we can improve responses to immunotherapy in patients with tumors that are predisposed to resistance.
Preclinical evidence suggests that radiation therapy (RT) could help circumvent immunotherapy resistance. When cancer cells are subjected to RT, they release antigens into the nearby environment, known as the tumor microenvironment (TME). The immune system can then recognize these antigens and tailor immune responses to the molecular “blueprint” of the cancer. In this way, RT may be able to enhance anti-tumor immune responses, both at the primary tumor treated with radiation and systemically, and at distant metastases far from the site of radiation. This effect is known as the abscopal effect of radiation (‘ab’ meaning away from, and ‘scopus’ meaning target).
These findings suggest that RT could enhance the response to immunotherapy, even at distant metastatic sites. However, this effect has not been consistently shown, especially in human biospecimens. To bridge this gap, we studied how lung cancers responded to immunotherapy in a phase 2 randomized clinical trial of patients with NSCLC receiving combination radioimmunotherapy. In one arm of this trial, patients received radiotherapy together with pembrolizumab, while in the control arm, patients received pembrolizumab alone.
To study each tumor’s response to therapy at the molecular and cellular level, our team analyzed 293 tissue and blood specimens derived from patients in this trial, from both pre-therapy and on-therapy timepoints. Using whole-exome sequencing and gene expression analyses, we identified tumors that harbored features of immunotherapy resistance and immunologically cold phenotypes. We were particularly interested in patients with this immunologically cold tumor phenotype and hypothesized that radiotherapy may circumvent immunotherapy resistance and sensitize such lung cancers to pembrolizumab. Below are the main insights from our research study.
We found that patients who received combination immunotherapy with RT showed enhanced anti-tumor immune responses, including in metastatic sites that were not irradiated. We examined gene expression patterns in the TME and observed an upregulation of key genes involved in inflammatory response and immune cell activation. This was also reflected in a greater number of T-cells, B-cells, macrophages, and natural-killer cells being recruited to the TME. As these phenomena were notes in tumors that harbored characteristics of immune resistance due to an immunologically cold TME, our findings supported a cold-to-hot transition of the TME, that we posit was driven by radiotherapy. This rewiring of the TME towards a more inflamed, immunologically “hot” phenotype enabled better clinical responses with immunotherapy.
Our second key finding was that, in the combination radioimmunotherapy arm, existing T-cells increased in density in both the blood and tissue compartments together with the appearance of new T cell clones. T-cells rely on T-cell receptors (TCRs) to recognize antigens, including tumor-associated and neoantigen-associated neoantigens. We employed T-cell receptor sequencing focusing on the antigen-recognizing domains to study changes in the T cell repertoire with radioimmunotherapy. Our hypothesis going into these analyses, based on our transcriptomic data, was that we would observe a reshaping of the T cell repertoire characterized by an influx and expansion of T cells in non-irradiated sites for patients who received radiation in addition to immunotherapy. Indeed, we found that patients who received RT in addition to immunotherapy exhibited an expansion of both existing and new TCRs, consistent with an enhanced immune response.
But were these T cells targeting the cancer cells? To answer this question, we cultured T-cells ex vivo from selected patients who attained long-term clinical outcomes with radioimmunotherapy despite their tumors having molecular features of resistance to immunotherapy. This led to our third key finding – that mutation-associated neoantigens elicited tumor-reactive clonotypic T cell expansions. This provided definitive evidence of the presence and enhancement of anti-tumor immune responses in the context of radioimmunotherapy.
Ultimately, when examining the clinical outcomes of patients who received radioimmunotherapy, we found that the phenomenon of the TME in non-irradiated metastatic sites warming up with radiotherapy was mainly observed in patients with the longest survival. Overall, patients who received radioimmunotherapy had significant tumor shrinkage at non-irradiated metastatic sites. This was especially evident in patients with tumors that initially exhibited an immunologically cold phenotype, and as a result, were not expected to benefit from immunotherapy.
Ultimately, our study highlights the potential application of RT in combination with immunotherapy for treating patients with NSCLC, particularly those with immunologically cold tumors who may otherwise have a poor response to immunotherapy alone. Taken together, our findings at the molecular, cellular, and systemic levels suggest that combination radioimmunotherapy may help circumvent immunotherapy resistance.
Our open-access study (DOI) was published in Nature Cancer on Tuesday, July 22nd, 2025.
May 16, 2025 | Blog Post
Significant advancements have been made in the therapeutic landscape of cancer, driven by the imperative to develop treatments for patients that are both effective and less toxic. Traditional therapeutic strategies like surgery, chemotherapy, and radiation therapy are generally effective but inherently limited by their lack of specificity regarding the molecular profile of tumors and collateral damage to healthy tissues. These constraints have spurred the pursuit of innovative approaches that leverage the body’s intrinsic defense mechanisms, collectively called immunotherapies. Immunotherapy activates the immune system to recognize and eradicate cancer cells and signifies a paradigm shift in oncology. By enhancing the body’s anti-tumor immune defense, immunotherapy has broadened the therapeutic arsenal and redefined the trajectory of cancer care, offering the potential for durable responses and improved patient outcomes.
The conceptual foundation of immunotherapy dates back over a century, with pioneering observations made by Dr. William Coley in the late 1800s. Coley noted tumor regression in patients with concurrent bacterial infections, suggesting that immune system activation could combat malignancies. Despite the initial promise, his methods, which involved administering bacterial toxins, lacked the mechanistic insights that modern science provides. Advances in immunology throughout the 20th century elucidated key components of the immune response, including T-cells and cytokines, and laid the groundwork for contemporary immunotherapeutic strategies. Checkpoint inhibitors emerged in the early 21st century; these block inhibitory pathways on T-cells that are a safety mechanism to prevent autoimmunity. Ipilimumab (which targets CTLA-4) and nivolumab/pembrolizumab (which target PD-1) showed that blocking immune checkpoints can reinvigorate anti-tumor immunity.
Immunotherapy encompasses several distinct modalities operating through different mechanisms. As mentioned above, checkpoint inhibitors disrupt inhibitory signals that cancer cells exploit to evade immune detection, thereby reinvigorating T-cell activity. Chimeric antigen receptor (CAR) T-cell therapy involves genetically engineering T-cells to express synthetic receptors that recognize tumor-specific antigens, enabling tumor clearance. Monoclonal antibodies bind to surface proteins on cancer cells, tagging them for destruction or blocking proliferative signaling. Cancer vaccines prime the immune system to recognize tumor-specific antigens, while cytokine therapies such as interleukin-2 and interferons amplify immune responses, enhancing tumor eradication.
Non-small cell lung cancer (NSCLC), the most common subtype of lung cancer, has experienced significant advancements due to immunotherapy. Drugs like pembrolizumab and nivolumab have become the standard of care, particularly for tumors with high PD-L1 expression. These therapies promote T-cell activation by disrupting the PD-1/PD-L1 axis, fostering more robust and sustained anti-tumor immune responses. Clinical trials have demonstrated that immunotherapy often surpasses chemotherapy in both efficacy and tolerability, representing a paradigm shift in the management of NSCLC.
Although immunotherapy has achieved notable successes, its effectiveness is inconsistent across all cases, making real-time monitoring of therapeutic responses essential for optimizing clinical outcomes. Traditional imaging techniques may fall short in promptly and accurately portraying therapeutic responses, especially in patients with heterogeneous responses or stable disease. Liquid biopsies have emerged as a minimally invasive alternative, analyzing circulating tumor DNA (ctDNA) molecules shed by tumor cells into the bloodstream. Longitudinal tracking of fluctuations in ctDNA levels enables clinicians to assess systemic tumor burden and treatment efficacy precisely. These approaches can elucidate the heterogeneity of immunotherapy responses and facilitate timely therapy modifications. Concurrently, monitoring T-cell activation and differentiation patterns provides insights into the immune system’s engagement, revealing proliferation, exhaustion, and memory formation markers correlating with differential therapeutic outcomes or the emergence of immune-related toxicities.
In conclusion, immunotherapy has redefined the field of oncology, harnessing the immune system to achieve targeted and durable anti-tumor responses. Its success is exemplified by breakthroughs in NSCLC treatment, where checkpoint inhibitors have improved clinical outcomes compared to traditional chemotherapy for many patients. However, timely and accurate capture of therapeutic response remains a challenge. Liquid biopsies and immune cell population profiling represent pivotal advancements in combating this challenge. Ongoing research is honing these technologies, enabling synergies between immunotherapy and real-time molecular monitoring of treatment response, ultimately leading to improved outcomes with precision immuno-oncology.
References:
- Pardoll, D.M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252-264.
- Topalian, S.L., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New England Journal of Medicine, 366(26), 2443-2454.
- Ribas, A., & Wolchok, J.D. (2018). Cancer immunotherapy using checkpoint blockade. Science, 359(6382), 1350-1355.
- June, C.H., O’Connor, R.S., Kawalekar, O.U., Ghassemi, S., & Milone, M.C. (2018). CAR T cell immunotherapy for human cancer. Science, 359(6382), 1361-1365.
- Aredo J.V. et al. Liquid Biopsy Approaches for Cancer Characterization, Residual Disease Detection, and Therapy Monitoring. Am Soc Clin Oncol Educ Book 45, e481114 (2025). DOI:10.1200/EDBK-25-481114
- Anagnostou, V., Ho, C., Nicholas, G. et al. ctDNA response after pembrolizumab in non-small cell lung cancer: phase 2 adaptive trial results. Nat Med 29, 2559–2569 (2023). https://doi.org/10.1038/s41591-023-02598-9
May 16, 2025 | Blog Post
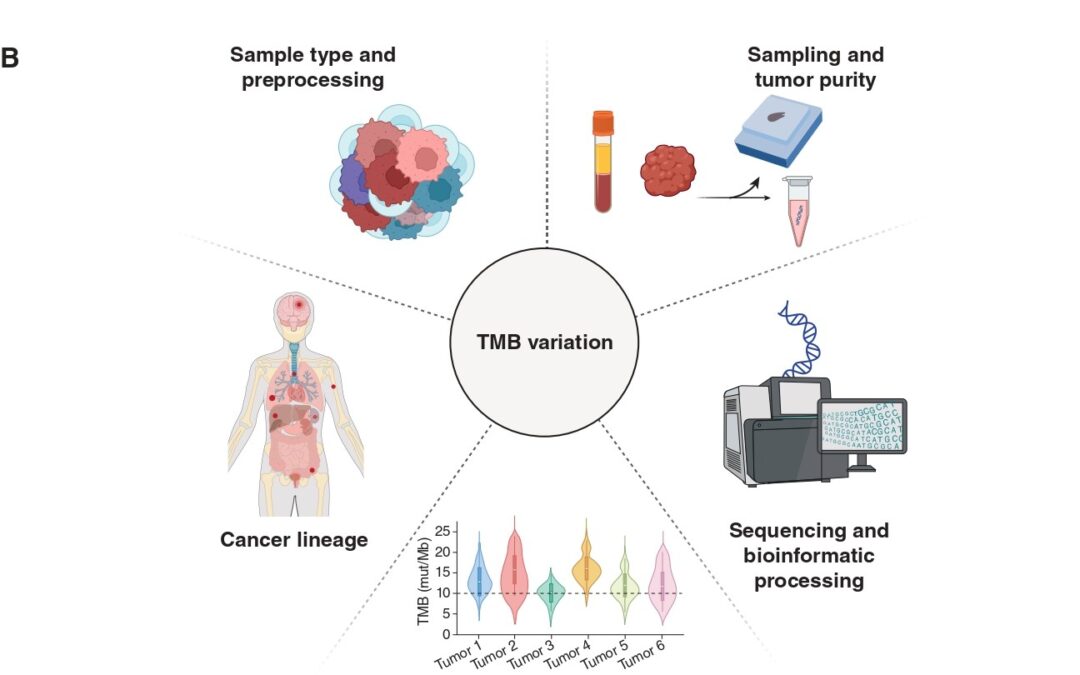
Immunotherapy, in the form of immune checkpoint inhibition (ICI), has transformed cancer care across multiple tumor types. However, existing biomarkers often fail to accurately predict which patients will draw maximal benefit. Tumor Mutation Burden (TMB) has emerged as a tumor-agnostic predictive biomarker of immunotherapy response. Measured as the number of somatic mutations per megabase (mut/Mb) of analyzed DNA sequence, TMB serves as a proxy of tumor “foreignness” to the immune system. The U.S. Food and Drug Administration (FDA) has granted approval of ICI with pembrolizumab for tumors with TMB ≥ 10mut/Mb regardless of cancer type. Despite this milestone, its clinical utility as a pan-cancer companion diagnostic for ICI is limited and TMB remains imperfect as it does not effectively and consistently predict immunotherapy response. In our recent publication (DOI), we revisit the biological, technical, and clinical nuances that limit TMB’s value and highlight how its fine-tuning might overcome these challenges.
The biological premise of TMB is straightforward: more mutations = higher chances of tumor immune recognition. But this view oversimplifies a process with a high degree of complexity. Not all mutations translate into peptides, and not all peptides are immunogenic. Only a fraction of mutation-associated peptides, known as neoantigens, can be effectively presented on major histocompatibility complex (MHC) molecules on cell surface and be recognized by T-cell receptors. Hence, neoantigen quality matters more than mutation quantity. A tumor with high density of immunogenic mutation-associated neoantigens may therefore respond better to ICI than a tumor with higher TMB but poorer neoantigen quality.
TMB is a static snapshot, while cancer is a moving target. Over the course of tumor evolution, immunotherapy imposes selective pressure and bottlenecks, causing cancer clones harboring different TMB values to be eliminated or prevail through a process known as immunoediting. This Darwinian process can significantly alter tumor’s neoantigen landscape over time. Thus, TMB as a numeric or binarized value (high/low) falls short in capturing the dynamic nature of tumor evolution.
In addition, not all mutations carry the same significance when it comes to predicting immunotherapy responses. Some happen as early as the initial steps of carcinogenesis, are shared among many cancer clones and are termed as “clonal”. Others, known as “subclonal”, occur later, are shared only among few tumor cells and are more prone to loss over time. Taking a step beyond tumor architecture through a more functional scope, our group has previously shown that the genomic context of mutations plays its own role in shaping their likelihood of loss due to immunoediting. Mutations occurring in the multicopy state or found in haploid genomic regions are less likely to be eliminated. Mutations in multiple copies may be biologically protected from loss, while mutations in essential haploid genomic regions cannot be purged without compromising cell survival (DOI).
Another important consideration lies in the specimen and methodology employed to calculate TMB. Current FDA approval refers to tissue-based TMB calculation through next-generation sequencing (NGS) of a prespecified gene panel. However, this method is subject to biases related to tumor heterogeneity, sampling site, and tumor purity, especially in metastatic or heavily pretreated settings. In our piece, we touch upon the opportunities blood-based TMB estimation provides. Our paper discusses the emerging role of blood-based TMB estimation from circulating tumor-derived DNA fragments (ctDNA). Liquid biopsy offers a less invasive, real-time alternative that captures spatial and temporal heterogeneity, enabling real-time tracking of tumor mutational status throughout ICI treatment.
The approved cutoff of 10mut/Mb for ICI administration, although convenient, may not be universally applicable in the pan-cancer context. Tumor histology and lineage play a critical role in shaping mutation patterns and immune responses. For instance, malignant mesothelioma typically stands in the lower TMB spectrum, yet some patients respond to ICI. Alternative biomarkers, such as genome-wide loss of heterozygosity, may prove more useful in these contexts (DOI). Our published work includes an analysis of TMB values in correlation with ICI responses across several cancers, highlighting the discrepancies histology and lineage impose when it comes to establishing cutoffs that maximize the predictive value of TMB.
Furthermore, the overall tumor genomic landscape within which TMB is interpreted holds great significance. High TMB may result from mutagenic processes such as microsatellite instability or POLE/POLD1 mutations (ultra-mutated phenotype), which are associated with favorable ICI responses. Additionally, distinct mutational signatures, including those induced by ultraviolet light, tobacco smoke, or APOBEC enzymes, can influence immunogenicity. Finally, co-mutation patterns largely dependent on cancer lineage add an extra biological modifier of ICI response that needs to be considered when calibrating TMB through the lens of tumor biology. Example of such patterns include STK11/LKB1 mutations, which have been shown to dampen ICI response despite high TMB.
Overall, it is true that TMB is far from perfect when it comes to predicting ICI response. Looking into the future, its biological and technical standardization is expected to upgrade its predictive worth and allow for maximal leverage of its value in the clinical setting.

Jan 7, 2025 | Blog Post
Precision oncology is revolutionizing the way we treat cancer by tailoring therapies to the genetic makeup of individual tumors. As our understanding of cancer’s molecular biology continues to expand, therapies are becoming more targeted, effective, and individualized. This shift toward personalized medicine is gradually becoming the standard of care, offering hope for better outcomes in patients with advanced cancers.
Molecular Tumor Boards (MTBs), are multidisciplinary teams of experts that play a pivotal role in the application of precision oncology and the translation of research findings into clinical practice. The Molecular Tumor Board routinely reviews cases of individuals with advanced cancer and interprets the genomic data obtained through next-generation sequencing (NGS) to ultimately explore molecularly-informed treatment recommendations. These MTBs however, are most commonly available in tertiary cancer centers, making access to them still limited. That is why our team at the Johns Hopkins Molecular Tumor Board (JH MTB) recently published a case report (DOI) in The Journal of Clinical Oncology (JCO), to open up an educational discussion on the genomically informed treatment paradigm. This case report leverages a discussion held at our Molecular Tumor Board that highlights the important role of the MTB in the management of metastatic breast cancer, and it details the journey of a patient whose tumor developed resistance to targeted therapy, identified using NGS, and interpreted by the JH MTB.
We discussed the case of a young woman diagnosed with early stage hormone receptor–positive, HER2-negative breast cancer, who as per standard of care, received neoadjuvant chemotherapy followed by bilateral mastectomy. Germline testing identified a heterozygous pathogenic loss-of-function PALB2 frameshift mutation which is known to be associated with an increased lifetime risk of breast, pancreatic, and ovarian cancer. The patient’s cancer recurred four years after the initial diagnosis and as biopsy of a metastatic site showed hormone receptor–positive breast cancer, she received letrozole monotherapy. Following further disease progression 15 months later she received the PARP inhibitor olaparib as part of a clinical trial given the presence of the PALB2 germline mutation.
PALB2 is one of the genes involved in DNA repair, specifically in homologous recombination (HR). Loss of function of PALB2 leads to the inability to properly repair DNA, specifically double-strand breaks, which can result in genetic instability and contribute to cancer progression. This is referred to as homologous recombination deficiency (HRD), and, as in this case, it can be driven by loss-of-function mutations in one of the genes of the HR pathway. Cancers with DNA repair deficiency rely on alternate DNA repair mechanisms and this creates an opportunity for therapies that block these alternate DNA repair pathways such as PARP inhibitors (PARPi) to block DNA repair in cancer cells ultimately leading to tumor cell death.
However, some tumors can develop resistance to PARPi through acquired mutations that restore homologous recombination, like in this case. At the time of disease progression on olaparib, a liquid biopsy detected the germline PALB2 mutation, together with a plethora of new PALB2 mutations that all restored the PALB2 reading frame, practically negating the effect of the germline loss-of-function mutation. The JH MTB reasoned that these reversion mutations all restored homologous recombination that allowed the metastatic clones to escape synthetic lethality through PARPi therapy. A large number of different PALB2 reversion mutations were detected in the bloodstream, which represents the reservoir where circulating DNA from any metastatic site is shed. As such, the JH MTB team hypothesized that different PALB2 reversion mutations were acquired and selected for in various metastatic sites and potentially at different timepoints during PARPi therapy. This essentially means that these reversion mutations, although all driven by a shared selective pressure of PARPi therapy, emerged separately at different metastatic sites and at different times during tumor evolution.
The emergence of polyclonal reversion mutations in PALB2, following PARP inhibition, suggest convergence evolution in this tumor, through independent genomic events, in response to the shared selective pressure of target therapy, ultimately restoring homologous recombination and DNA repair, and leading to a clinically refractory phenotype. Such reversion mutations have been observed in ovarian, breast, pancreatic, and prostate cancer with a prevalence of approximately 26% and the cancer clones harbouring these reversion mutations gain a fitness advantage, followed by positive selection that clinically manifests as therapy resistance. As seen in this case, different subclones can develop genomically distinct but phenotypically similar resistance when subjected to a shared selective pressure.
This case highlights the importance of elucidating the underlying mechanism of resistance and uncovering the molecular biology driving the refractory phenotype. By leveraging advanced genomic techniques like next-generation sequencing and the multi-disciplinary expertise of Molecular Tumor Boards, we can uncover the intricate mechanisms behind treatment resistance, such as the polyclonal reversion mutations observed in this patient. Our findings also illustrate the importance of real-time monitoring to adapt treatment approaches. As personalized therapies continue to evolve, the integration of genomic profiling into clinical decision-making remains essential for identifying the most effective treatment options and overcoming resistance. This case additionally highlights the advantage of liquid biopsy to both allow for minimally invasive real-time serial monitoring for arising resistance variants and to enable comprehensive assessment of the genomic landscape across metastatic sites in heterogeneous tumors. The collaborative, multidisciplinary MTB review process exemplified by this case is a powerful model for translating molecular insights into actionable treatment strategies, ultimately improving outcomes for patients with advanced cancer. Lastly, we emphasise the need for broadening access to MTBs, ensuring that genomically informed care is available to a wider range of patients.






