![]() Posing hypothesis-driven and clinically relevant questions represents one of the pillars of scientific discovery that can be translated in interventions that may improve patient outcomes. And that’s exactly what our research team was able to do with BR.36; a multi-center, randomized, ctDNA-directed, phase 2 trial of molecular response-adaptive immuno-chemotherapy for individuals with lung cancer. Recently published in Nature Medicine (DOI), and building on years of work on minimally invasive liquid biopsies in capturing therapy response to immunotherapy, our group formed, asked, and investigated the clinical value of using a molecular readout of therapy response during immunotherapy.
Posing hypothesis-driven and clinically relevant questions represents one of the pillars of scientific discovery that can be translated in interventions that may improve patient outcomes. And that’s exactly what our research team was able to do with BR.36; a multi-center, randomized, ctDNA-directed, phase 2 trial of molecular response-adaptive immuno-chemotherapy for individuals with lung cancer. Recently published in Nature Medicine (DOI), and building on years of work on minimally invasive liquid biopsies in capturing therapy response to immunotherapy, our group formed, asked, and investigated the clinical value of using a molecular readout of therapy response during immunotherapy.
Evaluation of therapy response on immunotherapy represents a challenge. Imaging that is predominantly used to evaluate cancer’s response to treatment, often falls short in rapidly and accurately capturing the therapeutic effect. The limitations of imaging are intensified in patients with radiographic stable disease or mixed responses, both representing heterogenous and hard to assess patient groups. In addition, already existing snapshot tumor-based biomarkers such as PD-L1 expression and tumor mutation burden (TMB), that can help guide therapy selection, are imperfect and inconsistently predict clinical outcomes with immunotherapy.
That’s where liquid biopsies and analyses of cell-free circulating tumor DNA (ctDNA) have gained momentum and can be particularly informative. As cells in our body die, they shed fragments of their DNA in the bloodstream. Cancer cells do so as well and these cell-free circulating tumor DNA remnants can be picked up by liquid biopsies (LB). From a LB assay standpoint, cell-free DNA is isolated from blood, followed by ultra-sensitive techniques that allow us to focus on specific regions of DNA and look for changes in the genetic code, known as mutations. These minimally invasive, innovative approaches allow for real-time tracking of circulating tumor burden, thereby molecularly assessing cancer’s response to treatment.
After identifying mutations in ctDNA, we are faced with the challenge of deciphering which of the mutations detected by the LB are truly tumor-derived. Here comes another challenge, that of identifying which mutations are coming from cancer cells and which ones come from other tissues and as such represent “biological noise”. These confounding mutations can be germline, meaning mutations passed down from previous generations, or can be originating from blood cells, which is termed “clonal hematopoiesis” (CH). To solely focus on tumor-derived mutations in our study, we used matched normal DNA genomic data, obtained through next generation sequencing of white blood cells (WBCs), which in turn allowed for filtering out CH-derived and germline mutations.
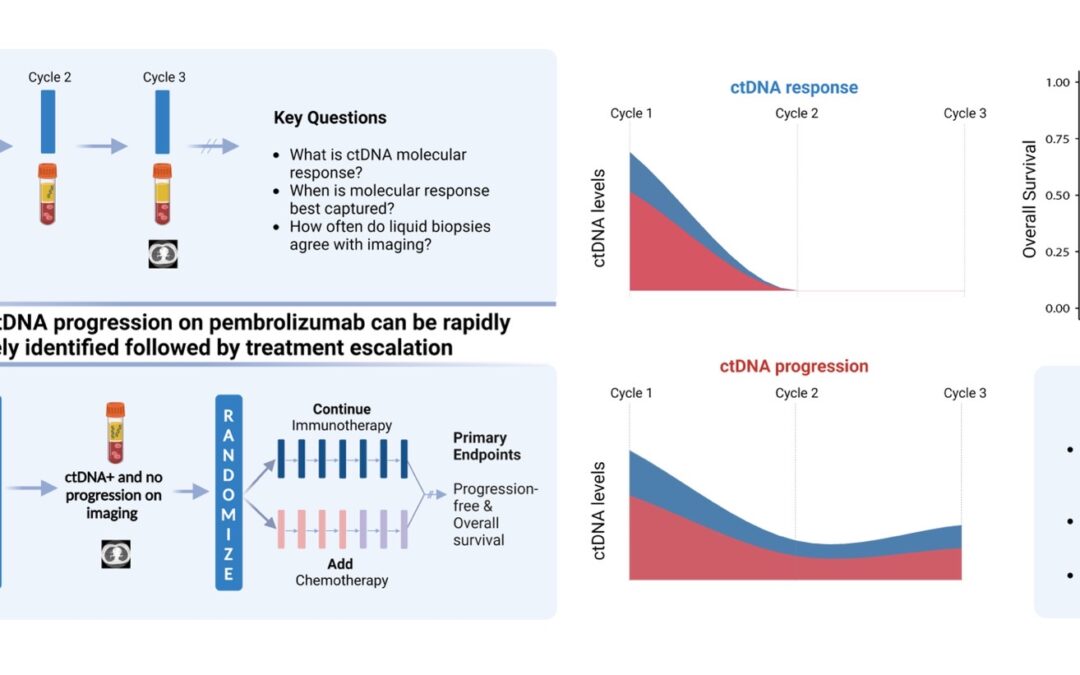
With that background in mind, let us dive deeper into the fundamental questions posed. Starting with the definition: What is ctDNA response? How soon after immunotherapy initiation should we look for it? How concordant is ctDNA molecular response with imaging response? And how does it relate to survival and clinical outcomes? Are there specific patients that would benefit most from a molecular assessment of their cancer’s response?
Let us rewind and unfold these one by one. Fifty patients with metastatic non-small cell lung cancer (NSCLC) were enrolled in the first observational phase of our trial and received immunotherapy as standard of care. LBs were performed with each of the first three cycles of immunotherapy and ctDNA load was estimated. Molecular response (mR) was defined as clearance of ctDNA mutation levels, whereas persistence or rise of ctDNA levels was classified as molecular disease progression (mPD). Our findings reveal that the median time necessary for achievement of mR was 2.1 months, or in other words, after 2 cycles of immunotherapy. We found that most patients with radiographic complete (CR) or partial (PR) response prior to cycle 3 of immunotherapy had mR on their liquid biopsy testing. We additionally and importantly found a strong correlation between molecular response and improved clinical outcomes, as patients in the mR group attained longer overall survival (OS) and progression-free survival (PFS) than patients showing mPD.
These findings suggest a unique actionable opportunity to use molecular response assessment to more optimally decipher the true therapy response including the heterogeneous group of patients with radiographically stable disease. Molecular responses were largely concordant with radiographic responses, however the former were more informative and effective in predicting clinical outcomes and survival.
Upon investigating these questions, what is next? Clinical translation. Eager to implement the aforementioned findings in clinical practice, we designed the second phase of BR.36; a ctDNA molecular response-driven interventional randomized stage of the trial that will assess the value of ctDNA molecular response in guiding therapeutic decision making (NCT04093167) and inform which patients should receive immunotherapy monotherapy vs. a combination of immunotherapy with chemotherapy. Looking at the expanding landscape of immunotherapy treatments for individuals with metastatic lung cancer, our vision is that ctDNA molecular response can rapidly and accurately predict therapy response and allow us to navigate treatments, tailoring therapies to the right patient populations at the right time.
![]()
Interested in more reading? Here’s a list of liquid biopsy studies from our group:
Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor relatlimab in resectable esophageal/gastroesophageal junction cancer: a phase Ib trial and ctDNA analyses. (DOI)
Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. (DOI)
Elucidating the Heterogeneity of Immunotherapy Response and Immune-Related Toxicities by Longitudinal ctDNA and Immune Cell Compartment Tracking in Lung Cancer. (DOI)